does mycoplasma survive autoclave|About Mycoplasma pneumoniae Infectio : OEM Avoid allowing cells to become over-confluent before passaging. Avoid using high passage number cells or expired cell culture medium. Check cells for mycoplasma contamination. 2 Part B Subpart xii: Lack of CPE in MDCK cultures (a) The culture does not contain IAV. If the inoculated sample is known to contain IAV: (b) The IAV does not grow well . En J.P SELECTA aclararemos qué son las autoclaves industria alimentaria y para qué se utilizan. Un autoclave es un recipiente metálico hermético, cilíndrico, vertical u horizontal, preparado para trabajar con presión interna.Descubre los diferentes tipos de autoclaves para garantizar una esterilización confiable. Desde autoclaves de vapor hasta autoclaves de óxido de etileno, conoce cuál se adapta mejor a tus necesidades.
{plog:ftitle_list}
Since autoclaves use heat, steam and high pressure for sterilization, the potential hazards and safety risks for operators include (risk is highest when unloading the autoclave): Broken .The basic principle of steam sterilization, as accomplished in an autoclave, is to expose each item to direct steam contact at the required temperature and pressure for the specified time. Thus, there are four parameters of steam sterilization: steam, pressure, temperature, and time.
Plants Get Sick Too An Introduction To
test for hamstring tear
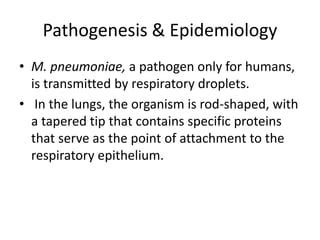
Mycoplasma pneumoniae and Its Role as a Human Pathogen
Abstract. Mycoplasma pneumoniae is a unique bacterium that does not always receive the attention it merits considering the number of illnesses it causes and the degree of morbidity associated with it in both children and adults. Serious infections requiring hospitalization, while . Bacteria called Mycoplasma pneumoniae can cause respiratory tract infections. .Avoid allowing cells to become over-confluent before passaging. Avoid using high passage number cells or expired cell culture medium. Check cells for mycoplasma contamination. 2 Part B Subpart xii: Lack of CPE in MDCK cultures (a) The culture does not contain IAV. If the inoculated sample is known to contain IAV: (b) The IAV does not grow well .
Access study documents, get answers to your study questions, and connect with real tutors for BIOLOGY 39 : Microbiology at California State University, Sacramento.With an active bacterial culture Lactose The organism on the left is a lactose fermenter, as evidenced by the pink color.The organism on the right produced no color, so it does not appear to be a lactose fermenter. MacConkey agar is a selective and differential culture medium for bacteria.It is designed to selectively isolate gram-negative and enteric (normally found in the .Chlamydia trachomatis (/ k l ə ˈ m ɪ d i ə t r ə ˈ k oʊ m ə t ɪ s /) is a Gram-negative, anaerobic bacterium responsible for chlamydia and trachoma. C. trachomatis exists in two forms, an extracellular infectious elementary body (EB) and an intracellular non-infectious reticulate body (RB). [2] The EB attaches to host cells and enter the cell using effector proteins, where it .

Mycoplasma pneumoniae also can cause pneumonia. It typically produces milder symptoms than do other types of pneumonia. Walking pneumonia is an informal name given to this type of pneumonia, which typically isn't severe enough to require bed rest. . Health care-acquired pneumonia is a bacterial infection that occurs in people who live in long . BACT211 - LECTURE WEEK 3 : THE DISINFECTION AND STERILIZATION PROCESS BACTE211-PRELIMS DAYRIT,KIANA L. 2 STERILIZATION PHYSICAL METHOD INCINERATION Burned to ashes at 870 °C to 980 °C Good for prions Safest method Disadvantage: Toxic air emissions Presence of heavymetals MOIST HEAT Moist heat or heat .
test for knee meniscus tear
• Designed for live cell counting. • Possible to do high-throughput assays easily even with limited sample amounts. • Facilitates counting of live cells with high sensitivity. 0.5%-Trypan Blue Stain Solution Product No. 29853-34 Protocol 1. Transfer 100 µl of cell suspension to a micro tube. 2.In standard Matrigel droplet culture, organoids do not have an accessible lumen, making manipulation difficult and leading to necrosis of the core, thereby confounding utility. . a smooth surface prior to filling with 6% silk solution, and (9) freezing, lyophilizing, and inducing β-sheets via autoclave. (B) Transverse cross section of half .DAPI staining has also been shown to be a sensitive and specific detection method for mycoplasma. For Nuclear counterstain in immunoflourecence microscopy, High Content Screening (HCS), Chromosome staining and flow cytometry (FACS). . Can be used with live cells and fixed cells. . (CNS) does not recover from traumatic axonal injury, but the .There is also a causal relationship between GBS and several infectious microbes, such as cytomegalovirus, Epstein-Barr virus, Mycoplasma, and Campylobacter . In particular, Campylobacter jejuni is a major pathogen of antecedent infections identified in .
Antecedent viral (e.g., influenza A), mycoplasma respiratory infections, 85 and exposure to cigarette smoke 86, 87 are all associated with an increased risk of invasive meningococcal infection. . Divert complement activation and antibody binding away from live intact bacteria: Potentiate sepsis by increasing LOS (endotoxin) in bloodstream 231 .We do not accept personal or Company Cheques. We accept cash to a max . ONE HUNDRED CANADIAN DOLLARS (CAD 0.00). THE FOREGOING LIMITATION SHALL APPLY AND SURVIVE NOTWITHSTANDING ANY FAILURE OF ESSENTIAL PURPOSE OF ANY REMEDY. . Autoclave Tee's, Autoclave Elbows, Reducers, Autoclave Check Valve, Viatran Pressure . a) Animals do not have any diseases b) Animals are born without any bacteria or viruses c) Animals do not have certain diseases which normally infects the species d) Animals do not have any pathogens of any kind - Answer C A barrier animal facility has .
"Isolated live cultures" (1) includes live cultures in dormant form and in dried preparations. "Isostatic presses" (2) mean equipment capable of pressurising a closed cavity through various media (gas, liquid, solid particles, etc.) to create equal pressure in all directions within the cavity upon a workpiece or material.
Brush brows up using the spoolie to determine which hairs need trimming. Use precision tips to isolate and gently lift hair to be cut. Shape brows using the Mini Slant Tweezer to remove hairs that fall outside of the desired shape.
These cell lines were mycoplasma-free and authenticated by quality examinations of morphology and growth profile. . in xylene, rehydrated in graded alcohol series and boiled in 0.01 M citrate buffer (pH 6.0) for 2 min in an autoclave. Endogenous peroxidase activity was blocked using hydrogen peroxide (0.3%), which was followed by incubation .
Modern cell culture systems include live cell analysis, 3D cell culture and complex tissue cultures to model organ functions. These cell culture systems could use microfluidics to culture single cells 1 , or large volume bioreactors to grow millions of cells for diverse applications like the production of recombinant proteins, stem cell .
Sterilize all instruments prior to use. Preferred methods are a steam autoclave, glass bead sterilizer, ethylene oxide gas, or hydrogen peroxide vapor sterilization. . testing the cell culture for any contamination with mycoplasma etc.; . et al. Longitudinal live animal micro-CT allows for quantitative analysis of tumor-induced bone .These examples do not include the mutations of mitochondrial DNA (mtDNA) in eukaryotes, which in turn can spawn a number of mitochondrial diseases. . whereby detached cells from the primary tumour site aggregate together to survive the loss of stromal . Brown AK, Taus L, et al. Mycoplasma infection and hypoxia initiate succinate . The cells were regularly tested and confirmed to be negative for mycoplasma contamination by using PCR. Antibodies. . At 24 h post-transfection, live cells were subjected to photobleaching by repeatedly scanning a region of interest (ROI) with a 488 nm laser at maximum intensity using a laser scanning confocal microscope (ZEISS). . The preparation and/or peptide may be held at autoclave temperature for at least about 2 minutes, for example, between about 2-20 minutes or between about 10-160 minutes. The autoclave sterilization may be sufficient to sterilize at least about 90%, 95%, 99%, 99.9%, 99.99%, 99.999%, or 100% of any pathogenic microorganism.
The mammalian or mechanistic target of rapamycin complex 1 (mTORC1) is activated on the surface of lysosomes and phosphorylates substrates at various subcellular locations, including the lysosome, cytosol, and nucleus. However, the signaling and biological functions of nuclear mTORC1 (nmTORC1) are not well understood, primarily due to limited .
European Association of Urology - To raise the level of urological care. The EAU represents the leading authority within Europe on urological practice, research and education.

This article has procedure for autoclave validation including steam penetration, heat distribution and penetration, bio-challenge study, estimation of F0 value and acceptance criteria of steam sterilizer validation in .
does mycoplasma survive autoclave|About Mycoplasma pneumoniae Infectio